Abstract
Introduction: CD19 is expressed on the cell surface of many types of non-Hodgkin lymphoma (NHL), including follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). ADCT-402 (loncastuximab tesirine) is an antibody drug conjugate (ADC) comprising a humanized antibody (Ab) directed against human CD19 conjugated to a pyrrolobenzodiazepine (PBD) dimer toxin. Loncastuximab tesirine (Lonca-T) has demonstrated potent anti-tumor activity against CD19-expressing B-cell malignancies in preclinical models. Interim results of the first-in-human clinical study of Lonca-T in the difficult-to-treat relapsed/refractory (R/R) NHL setting are reported here.
Methods: Patients (pts; ≥18 years of age) with R/R B-cell lineage NHL who have failed or are intolerant to established therapies, or have no other treatment options available, are being enrolled in this Phase 1, multicenter, open-label, two-part study. The primary objectives for Part 1 (dose escalation) are to evaluate the safety and tolerability of Lonca-T, and to determine the maximum tolerated dose (MTD) and the recommended dose(s) to use in Part 2 (dose expansion). The primary objective for Part 2 is to evaluate the safety and tolerability of the dose(s) determined in Part 1. Efficacy (measured by overall response rate [ORR], duration of response [DoR], progression-free survival [PFS] and overall survival), pharmacokinetics, pharmacodynamics, and other exploratory endpoints are also being assessed in both parts of the study. Pts receive 1-hour intravenous infusions of Lonca-T every 3 weeks (1 cycle) according to a 3+3 dose-escalation study design. No intra-patient dose escalation is allowed.
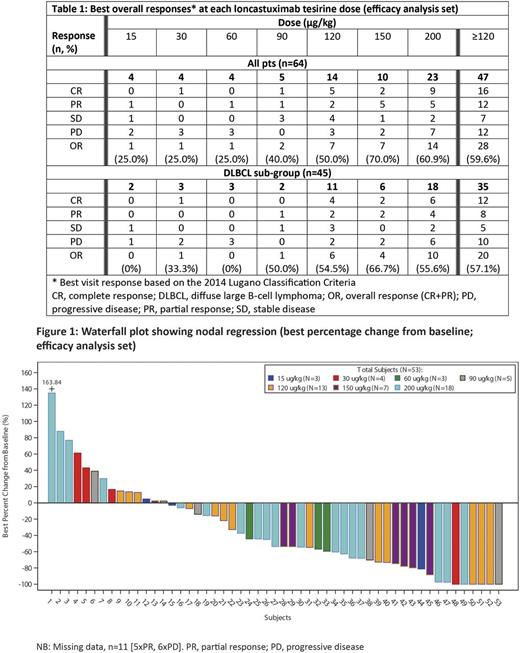
Results: As of July 5, 2017, 80 pts (55 male, 25 female; median age: 65.5 years [range 24-85]; median number of previous therapies: 3 [range 1-10]) have been recruited. Diagnoses were DLBCL (n=56), mantle cell lymphoma (MCL; n=9), FL (n=6), marginal zone B-cell lymphoma (n=3), chronic lymphocytic leukemia (n=2) and other (n=4). Pts have received doses of Lonca-T ranging from 15 to 200 µg/kg (median cycles: 2 [range 1-16]). Treatment-emergent adverse events (TEAEs) were reported in 76 (95.0%) pts, and grade ≥3 TEAEs in 46 (57.5%) pts. The most common non-hematological all-grade TEAEs were fatigue (35 [43.8%] pts), peripheral edema (21 [26.3%] pts) and nausea (20 [25.0%] pts), and grade ≥3 TEAEs were increased gamma-glutamyltransferase (7 [8.8%] pts) dyspnea (4 [5.0%] pts) and fatigue (4 [5.0%] pts). Hematological abnormalities included decreased hemoglobin (74/77 [96.1%] and 9/77 [11.7%] pts with all-grade and grade ≥3 TEAEs, respectively), decreased neutrophil count (42/69 [60.9%] and 29/69 [42.0%] pts, respectively) and decreased platelet count (55/77 [71.4%] and 21/77 [27.3%] pts, respectively). TEAEs in 8 (10.0%) pts led to treatment withdrawal (increased gamma-glutamyltransferase [n=4]; increased blood alkaline phosphatase [n=1]; periorbital edema [n=1]; fatigue [n=1]; abdominal pain [n=1]; thrombocytopenia [n=1]). A dose-limiting toxicity (DLT) was reported in 1 pt (worsening of thrombocytopenia at 200 µg/kg) and the MTD has not yet been reached. Table 1 shows the best overall response by dose. At doses ≥120 µg/kg, 16/47 (34.0%) and 12/47 (25.5%) evaluable pts achieved a complete response (CR) or partial response (PR), respectively (ORR: 28/47 [59.6%]). ORR for pts with DLBCL was 20/35 (57.1%), with a CR rate of 34.3% (12/35). Figure 1 depicts the waterfall plot. Pharmacokinetic measures for total- and PBD-conjugated Ab exposures were comparable, were proportional to dose, and were associated with modest accumulation by Cycle 2. Measures for unconjugated PBD were predominantly below quantification levels for all doses and time points. Associations of exposure to safety and efficacy are reported in a companion abstract (O O'Connor et al.).
Conclusions: In this Phase 1 study, Lonca-T has demonstrated encouraging single-agent anti-tumor activity and manageable toxicity in pts with R/R B-cell lineage NHL. One DLT has been reported and the MTD has not yet been reached. Evaluation in specific NHL subtypes is now warranted, and a dose expansion in pts with DLBCL is planned initially. Updated safety, tolerability, and efficacy results will be presented at the meeting.
Study sponsored by ADC Therapeutics. http://clinicaltrials.gov/show/NCT02669017
Kahl: ADC Therapeutics: Research Funding; Celgene: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Genentech: Consultancy. Hamadani: Takeda, Otsuka, MedImmune, Merck, ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; Celgene, Cellerant, Jansen, MedImmune: Consultancy. Caimi: Seattle Genetics: Equity Ownership; Incyte: Equity Ownership; Abbvie: Equity Ownership; Celgene: Speakers Bureau. Carlo-Stella: ADC Therapeutics: Research Funding. Reid: ADC Therapeutics, Millennium: Research Funding. Feingold: ADC Therapeutics: Employment, Other: Potential equity interest. Ardeshna: ADC Therapeutics: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Conference Expenses; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Conference Expenses, Research Funding. Radford: Takeda: Research Funding, Speakers Bureau; Seattle Genetics: Speakers Bureau; Novartis: Speakers Bureau; GlaxoSmithKline: Equity Ownership; AztraZeneca: Equity Ownership; ADC Therapeutics: Research Funding. Solh: Celgene: Speakers Bureau; Amgen: Speakers Bureau; ADC Therapeutics: Research Funding. Chung: ADC Therapeutics: Research Funding. Heffner: ADC Therapeutics: Research Funding. He: ADC Therapeutics: Employment, Other: Potential equity interest. Boni: ADC Therapeutics: Employment, Other: Potential equity interest. O'Connor: ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.